ECHO
Title:
The Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial: A Multi-Center, Open-Label, Randomized Clinical Trial Comparing HIV Incidence and Contraceptive Benefits in Women using Depo Medroxyprogesterone Acetate (DMPA), Levonorgestrel (LNG) Implant, and Copper Intrauterine Devices (IUDs)
Acronym:
ECHO
Site Principal Investigators:
- Prof. Elizabeth Bukusi Principal Investigator
- Dr. Maricianah Onono Co- Principal investigator
- Dr. Stella
Timeline:
2014 – 2016
Status:
Completed
Funder:
Sponsor: Bill and Melinda gates Foundation.
Coordinated by: FHI 360
Objective:
The Evidence for Contraceptive Options and HIV Outcomes is an open-label randomized clinical trial that compared three highly effective, reversible methods of contraception to evaluate whether there is a link between use of any of these methods and increased risk of acquiring HIV infection.
Brief Description
The Evidence for Contraceptive options and HIV Outcomes is an open-label randomized clinical trial that will compare three highly effective, reversible methods of contraception to evaluate whether there is a link between use of any of these methods and increased risk of acquiring HIV infection.
Background
- Women worldwide need family planning, and in Africa, the use of hormonal contraception, and especially Depo, provide women with a long-acting, reversible and safe option for birth control.
More than 150 million women around the world use hormonal contraceptives. - African women are at high risk of HIV.
16 million women aged 15 years and older are living with HIV; 80% live in sub-Saharan Africa
Young women 15–24 years old in sub-Saharan Africa are twice as likely as young men to be living with HIV.
African countries with HIV prevalence also have high rates of women using hormonal contraception
- The reasons for this are unclear.
- There is confusing data about whether there is a link between using some contraceptives and an increased risk of contracting HIV.
Objectives
Primary objective
- To compare the risks of HIV acquisition between women randomised to DMPA, levonorgestrel (LNG) implants, and copper IUDs
Secondary and tertiary objectives
- Pregnancy, safety, contraceptive continuation
Why do we need the ECHO Study?
- For over 25 years, the world has lived with the uncertainty about whether or not use of hormonal contraceptives increases HIV risk.
- ECHO aims to answer this critical public health question of the possible risks (HIV acquisition) and benefits (pregnancy prevention) of the three commonly-used, effective contraceptive methods among women who desire contraception
Purpose of the ECHO Study
When comparing women’s use of the contraceptives— Depo, Jadelle and IUD:
- Is there an increased risk of acquiring HIV when they use one method over the others?
- Are there more or less side effects of each method?
- Are the pregnancy rates the same?
- How well do women stay on each of the three contraceptive methods?
ECHO Sites
The study will take place at 12 sites across Eastern and Southern Africa, including sites in:
- Kenya – Kisumu
- South Africa
- Swaziland
- Zambia
Who can participate in the ECHO study
- Sexually active women 16-35 years old
- HIV negative and willing to be tested
- Seeking effective contraception
- Do not want to become pregnant for the duration of study participation
- Willing to be randomised to any of the three contraceptives being tested
- Willing to give consent to participate
Voluntary and confidential
- All information shared with trial staff will kept confidential.
- Women are asked to be honest at all times in their answers to staff.
- Participation is voluntary and women may leave the study at any time they wish.
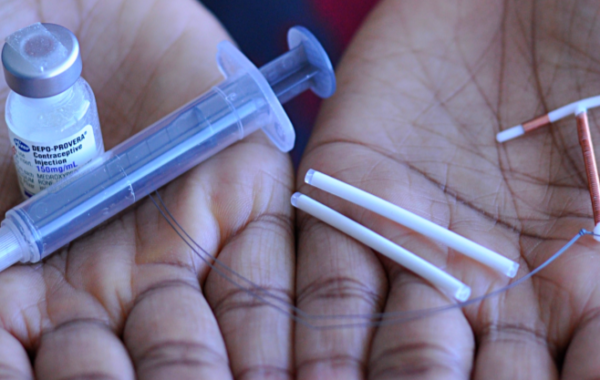
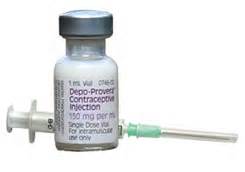
DMPA or Depo Provera
- Most widely used progestin-only injectable
- Given every 3 months as injection in arm
- Return of fertility is often delayed, by a minimum of four months
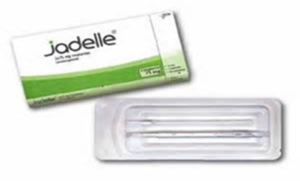
Jadelle Implant
- Consists of two thin, flexible rods filled with synthetic progestin that are inserted just under the skin of a woman’s upper arm
- Once inserted, lasts up to 5 years, although one can have it removed at any time Rapid return to fertility once removed
Copper IUD (Cu-IUD)
- The copper-bearing intrauterine device (Cu-IUD) is a small, flexible plastic frame with copper sleeves or wire around it that is inserted in the uterus (womb)
- Once inserted, lasts up to 10 years, although one can have it removed at any time
- Return to fertility is immediate

How the study works
Recruitment and Enrolling in the Study
- Potential participants will be invited to the trial site to learn about the study
- During an initial visit, they will learn about the risks and benefits and also about what is included in the visits (known as informed consent).
- Women will learn about the 3 contraceptives being tested and asked if they would be willing to use any of the 3 products
When a woman enrols in ECHO, she will be randomly placed in 1 of 3 groups
- Participants in all groups will be given the same standard prevention package (condoms, HCT, STI treatment)
- All women have an equal chance of being placed into each group.
- Neither she nor the staff can choose which product each participant will receive.
- Selection into a group is random, like rolling a dice.
- Once a participant is in a group, she will be encouraged to remain on her assigned method for the duration of the study.
Participant study visits schedule
What happens during study visits?
- Provide contraceptive counselling
- Provide HIV counselling and testing
- Ask questions about sexual behaviour
- Do a pregnancy test if needed
- Check health – for STIs and side effects to products
- Update contact information
- Schedule next appointment
- Give reimbursement for transport
